In this blog Dr Harvinder Virk (co-founder of the Only Good Antibodies community and forum) and Dr Nikki Osborne (founding director of Responsible Research in Practice) discuss the importance of antibody quality control.
Antibodies are widely used in biological sciences and drug development research to identify and isolate molecules of interest. The performance of an antibody will vary between applications, protocols, and cell/ tissue types. Partly because of this, the onus to confirm the appropriate performance of research antibodies is currently on the researcher, not the supplier. There are over 6 million commercial antibodies on the market1. Experiments that describe the performance qualities of antibodies are termed characterisation. Validation confirms the appropriate selectivity of an antibody (for the target) in the specific experiment of interest, for which there are consensus approaches2. We use the term quality controls to refer to characterisation or validation.
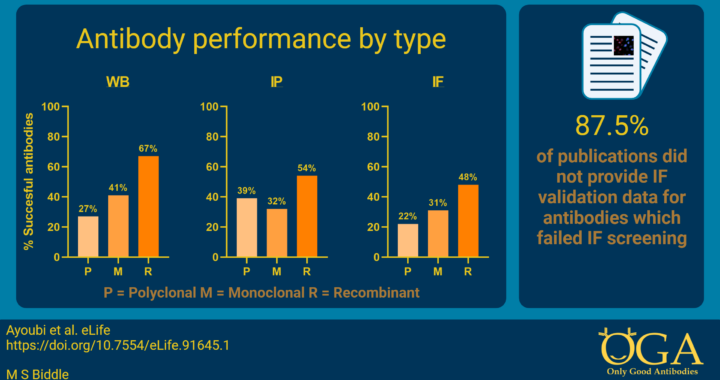
This complexity, and scale of antibody use, may have contributed to an antibody marketplace that contains a high proportion of poorly performing antibodies3-5. Our recent collaborative publication quantified the likely scale of the problem of poorly performing commercial antibodies used in the literature for the first time6. Greater than 50% of 614 antibodies failed characterisation experiments in three commonly used applications (western blotting, immunofluorescence, and immunoprecipitation). We found that for a set of 65 proteins, each protein was linked to an average of 12 published papers that presented data using poorly performing antibodies. Moreover, 87.5% of papers using poorly performing antibodies in one application (immunofluorescence) did not present any relevant quality control data (see Feature image). Add to this the findings of another study published in 2013 that only 44% of antibodies described within 200 biomedical research papers were uniquely identifiable7 and it becomes clear that there is room for improvement.
What can you do about it?
Antibody selection is often not based upon meaningful evidence, but it doesn’t need to be this way. Many purchasing decisions are currently informed by factors other than robust data about antibody performance. The Only Good Antibodies community is seeking to change this. By raising awareness of the challenges and supporting researchers to find and use, or generate and share quality antibody validation data change is possible.
To learn more join Dr Harvinder Virk and Dr Nikki Osborne on-demand for a FREE Responsible Research webinar discussing efforts to improve the accessibility and use of validated antibodies, plus what you can do to make a difference. To watch CLICK HERE
References:
- Longworth S, Chalmers A. CiteAb for researchers and suppliers: How identifying product citations from publications can help accelerate science. Information Services & Use. 2022;42(3-4):319-324.
- Uhlen M, Bandrowski A, Carr S, et al. A proposal for validation of antibodies. Nature methods. 2016;13(10):823-827.
- Baker M. Antibody anarchy: a call to order. Nature. 2015;527(7579):545-551.
- Baker M. Blame it on the antibodies. Nature. 2015;521(7552):274.
- Goodman SL. The antibody horror show: an introductory guide for the perplexed. New biotechnology. 2018;45:9-13.
- Ayoubi R, Ryan J, Biddle MS, et al. Scaling of an antibody validation procedure enables quantification of antibody performance in major research applications. eLife 2023 12:RP91645 https://doi.org/10.7554/eLife.91645.1
- Vasilevsky NA, Brush MH, Paddock H, et al. On the reproducibility of science: unique identification of research resources in the biomedical literature. PeerJ. 2013;1:e148.